OCR enabled management software to automate stability testing

Stability testing of active pharmaceutical ingredients and finished pharmaceutical products provides evidence on how the quality of the drug substance or product can vary under influence of various environmental factors. These include temperature, humidity, light, etc.
For the drug substance or product, stability testing is aimed to determine the period it is actually safe to use. This protects end consumers and assures the quality and efficiency of the ready-to-use medication.
Marketing authorization along with post-marketing studies must provide accurately maintained stability data. This way, they confirm the physical, chemical and microbiological properties of the new substance or product remain compliant within the expiry period.
Oftentimes, companies have difficulties with maintaining supportive documentation in an appropriate manner. And that’s when implementing computational technology, in particular OCR technology, can be game changing.
Legislative background
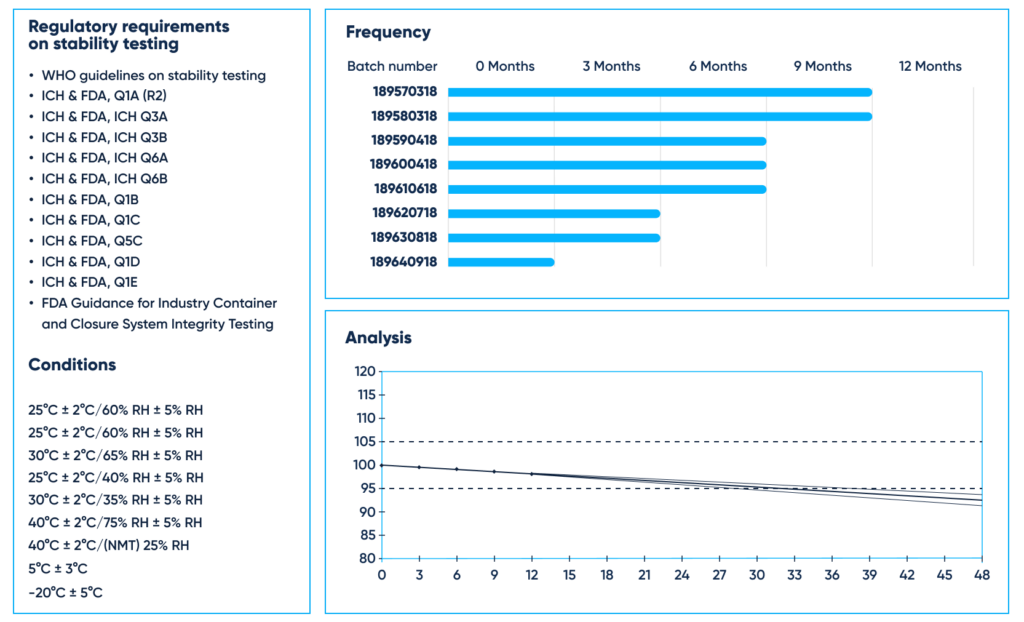
Stability testing of new drug substances and products is to be established in accordance with acknowledged regulatory requirements, such as:
- WHO guidelines on stability testing of active pharmaceutical ingredients and finished pharmaceutical products, Annex 10
- ICH & FDA, Q1A (R2) Stability Testing of New Drug Substances and Products
- ICH & FDA, ICH Q3A Impurities in New Drug Substances
- ICH & FDA, ICH Q3B Impurities in New Drug Products
- ICH & FDA, ICH Q6A Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Chemical Substances
- ICH & FDA, ICH Q6B Specifications: Test Procedures and Acceptance Criteria for New Drug Substances and New Drug Products: Biotechnological/Biological Products
- ICH & FDA, Q1B Photostability Testing of New Drug Substances and Products
- ICH & FDA, Q1C Stability Testing for New Dosage Forms
- ICH & FDA, Q5C Quality of Biotechnological Products
- ICH & FDA, Q1D Bracketing and Matrixing Designs for Stability Testing of New Drug Substances and Products
- ICH & FDA, Q1E Evaluation of Stability Data
- FDA Guidance for Industry Container and Closure System Integrity Testing in Lieu of Sterility Testing as a Component of the Stability Protocol for Sterile Products
The constraints that should be considered carrying out stability testing include the following restrictions:
Restriction 1
The studies must be carried out with consideration of the registration region in the Climatic Zones I, II, III, IV. These come with different condition prescriptions for the long-term or accelerated storage, in particular 30°C/65% RH or even more strict humidity conditions, for example, 30°/75% RH for more hot and humid areas or 25°C ± 2°C/60% for the general case.
Restriction 2
According to the recommendations of the regulatory authorities, supportive records for all critical attributes should be accurately presented in an appropriate format, for example, in tabular, graphical or narrative format. The evaluation of these should be included in summarizing documentation.
Other parameters to be reported include:
- Quantitative attributes (critical quality attributes (CQA) at all time points (assay, impurities, degradation products, preservative content, pH, dissolution) for new drug substances and products)
- The outcomes of the statistical analysis (linear regression, statistical modeling, poolability tests, covariance audit)
Restriction 3
It is also important to pay fair attention to analysis of the quantitative attribute that’s expected to change. The approach is to basically determine the point in time at which the 95% confidence limit for the mean curve crosses the acceptance criterion.
Restriction 4
If analysis shows the batch-to-batch variability, it’s beneficial to combine the data into one overall estimate. This can be done by primarily applying appropriate statistical tests to the varying slopes of the regression lines and the zero-time intercepts for the individual batches.
Small remark: The stability of new dosage forms might also be acceptable with a reduced database at the submission point:
- 6 months if accelerated
- 6 months long-term data from the ongoing studies
Restriction 5
Chromatographic fingerprints along with appropriate methods of assay by using marker substances laid down in the shelf-life specs relating to herbal substances and preparations, as well as traditional and non-traditional medicinal products.
Restriction 6
Tests validating container closure system integrity to demonstrate the capability of containers to continuously maintain sterile.
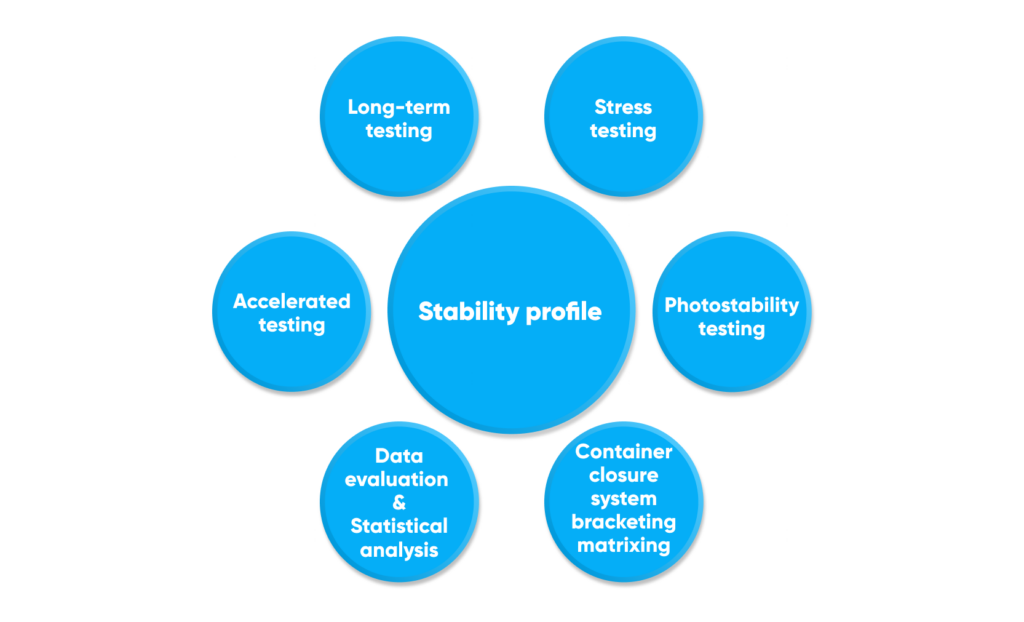
Primary data
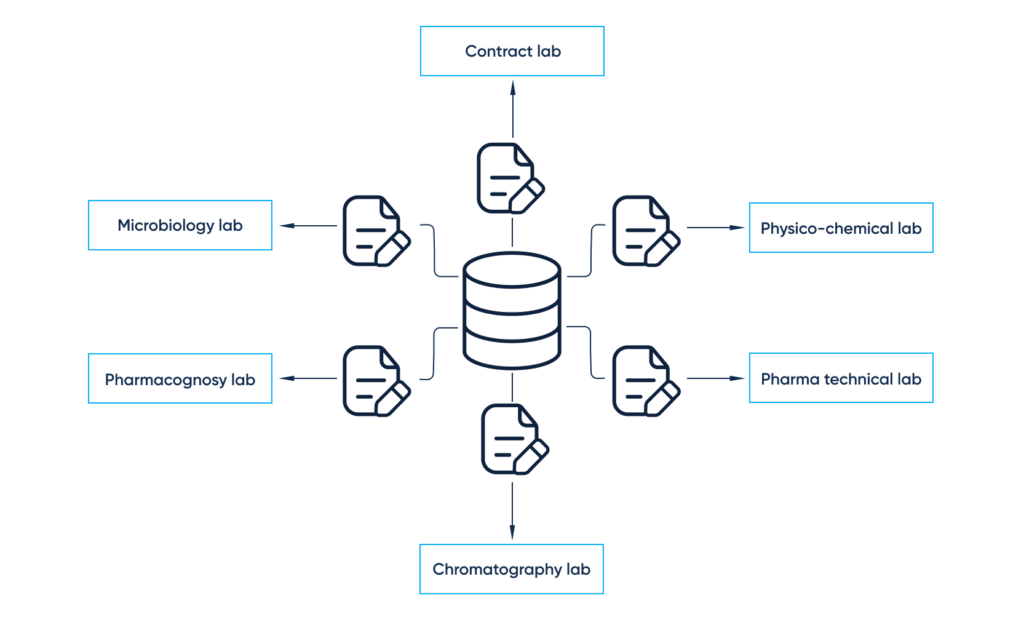
Primary data to support the recommended storage period for either drug substances or products, based on long-term, intermediate, accelerated testing, photostability as well as stress studies, are provided by subunits with specialties such as:
- Physical, physico-chemical methods (physical appearance, solubility, pH, free formaldehyde, total ash, loss on drying, residue on ignition, heavy metals, etc.)
- Chromatography (gas, thin-layer, high-performance liquid, related substances, enantiomeric purity, residual solvents, etc.)
- Pharmacognosy (water soluble extractive value, essential oils, bitterness value, etc.)
- Pharmaceutical technical lab (dissolution, disintegration, etc.)
- Biological, microbiological lab (total variable aerobic count, bacterial endotoxins, pyrogens, sterility, antigens, potency)
- External third-party labs (pesticide residues, aflatoxins, ochratoxins, determination of elemental impurities, surface resistance, arsenic release)
As for biotechnological and biological products, primary data (traditionally paper-based and manually filled out) is commonly obtained from:
- The physicochemical, biochemical, immunochemical analytical characterizations of the drug substance and/or product (molecular size, charge, hydrophobicity)
- The detection of any degradation changes by using:
- electrophoresis techniques (SDS-PAGE, immunoelectrophoresis, Western blotting, isoelectric focusing)
- high-resolution chromatography (reversed-phase chromatography, gel filtration, ion exchange, affinity chromatography)
- peptide mapping
Management system facilitated with OCR technology to digitize required record-keeping
Stability testing is fundamental for the accurate evaluation for shelf-life and recommended storage conditions. Retaining reliable supportive documentation means ensuring the quality and safety of both drug substances and finished drug products.
Stability data must contain:
- Results of stress testing
- Results of photostability testing
- A selection of batches supporting data
- Accurate information about container closure system
- The justification of proper storage conditions
- The estimation of results
- Specifications
- Statements
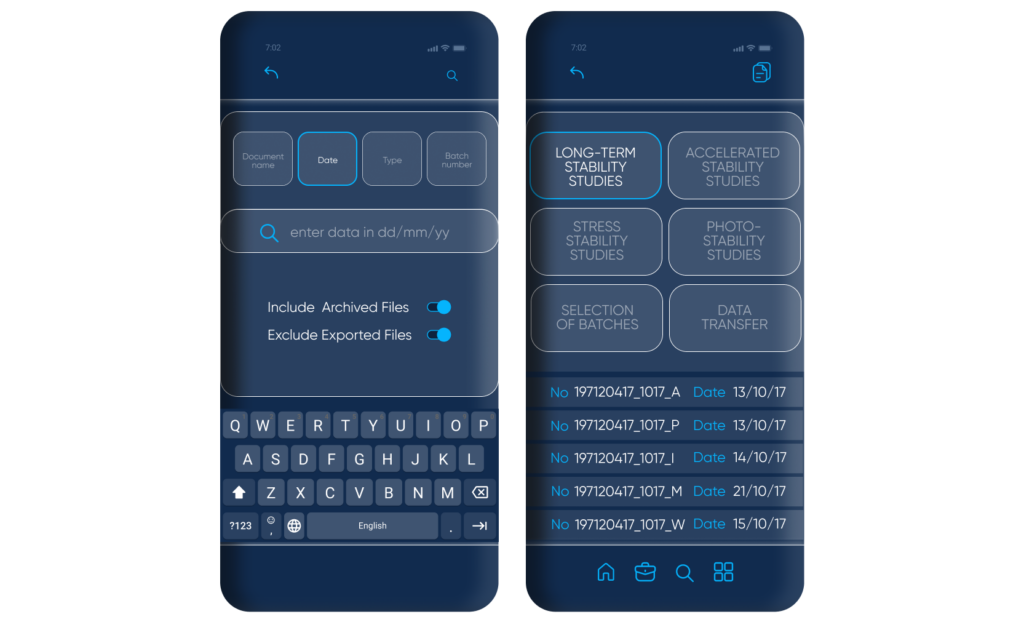
The introduction of an OCR solution can be extremely beneficial in terms of arranging collected information. With an optical character recognition solution, supportive documentation can be quickly scanned and digitized to be further transferred and analyzed.
OCR technology can help surmount difficulties such as:
- Source diversity
- Unproductive, behindhand, and resource-intense information transfer
- Human error
- Complicated consolidation
We see great opportunities in implementing OCR systems for strategic-thinking pharmaceutical organizations. By leveraging computational advances, business leaders can improve data gathering and its further processing, statistical analysis, modeling, reporting, and more.
Using custom OCR software, pharma companies can easily structure information and optimize daily workflows. This means, the managers can ensure that the data gathered is complete, consistent, accurate, and accessible to stakeholders.
Utilizing the right tools, these duties can be either facilitated or automated:
- Stability coordination
- Annual stability plan monitoring
- Regular stability protocol review and approval
- New stability study initiation and creation
- Completing stability study verification and closure
- Report maintenance
- Compliance assurance
- Quality analysis
- Obtaining data from the R&D and QC departments (physico-chemical, microbiological, pharmacognosy, chromatography, pharma-technical and contract labs)
- Communicating with R&D and QC departments in regard to new and ongoing stability studies
- Summarizing final stability reports
- Preparing final stability reports and tables
- Ensuring testing and analysis is completed on time to meet defined timelines
- Ensuring all stability studies are conducted in accordance to procedures and protocols
- Control analysis
- Preparing and maintaining documentation, including protocols and reports
- Transferring data within departments
The concept we suggest is an easy-to-navigate, reliable management platform with integrated OCR technology. It can be used to optimize operational processes, so that they meet the acknowledged industry requirements.
The suggested management platform with integrated OCR functionality can also provide for additional security. The features to consider might include user profiles, role-based access, and regular data back-ups.
The return on investment
When considering the implementation of a management platform with integrated recognition technology, pharmaceutical organizations will see how this groundbreaking decision might be strategically advantageous. The return of investment is expected in about 6 month, and might go beyond 1 million US Dollar in just 5 years of implementation.
For clarity, we made a forecast based on the average statistical data in the pharma industry. We compared several available on the market solutions which provide for stability testing automation.
| Solution 1 | Solution 2 | Solution 3 | Solution 4 | Abto Software’s solution concept | |
| Stability processing possibility | – | partly | – | partly | + |
| Licensing | + | + | – | + | – |
| Users quantity | 100 | 25 | multiple | 100 | multiple |
| OCR/paper forms input | – | – | – | – | + |
| Manual input | – | + | + | + | + |
| Annual price, $ | 39,600 | 38,200 | 50,000 | 276,750 | 153,460 |
| 5-year price, $ | 198,000 | 191,000 | 170,000 | 1,383,750 | 153,460 |
| 5-year cost saving with Abto Software (approximately) | 44,540 | 37,540 | 16,540 | 1,230,290 |
The right management software, especially with recognition functionality, facilitates smart resource allocation. Thought-out workloads, employee satisfaction, automatic detection of issues, immediate notifications, improved analysis and planning are just some factors that drive cost efficiency.
The cost of sticking to traditional, used routines, in particular paper-based records, is not always measurable. The time commonly spent on handwriting, transferring, processing, cutting, pasting, as well as validating (getting approval) is hard to calculate, but usually quite immense.
So, is it worthy to switch the strategy and automate operational processes, in particular daily record-keeping? For sure!
How we can help
Abto Software covers consulting, project management, software development, testing, maintenance, and more to deliver automation solutions that optimize operational processes, in particular day-to-day record-keeping. We focus on automation, cost efficiency, risk elimination, and performance.
And for the businesses rapidly growing, our company also integrates optical character recognition technology. We help our clients to seamlessly scan and transfer traditionally paper-based records to the central database to ensure gathered data is complete, consistent, accurate, and accessible.
The solutions we deliver:
- Business automation (ERP & CRM platforms)
- Telemedicine and telehealth solutions (for example, smart chatbots)
- Predictive analytics (enterprise tools for discovering data patterns and making accurate predictions)
- Recommendation systems (EMR & EHR systems)
Our expertise:
- Artificial Intelligence (ML, DL & ANN)
- Computer Vision
- Optical Character Recognition (OCR)
- Blockchain technology


