OCR tool for facilitated drug distribution

Wholesale distribution of finished pharmaceutical products is an intricate process with many special nuances. Transportation conditions, packaging integrity, temperature and humidity levels, the sanitization of premises, and other important details must be carefully inspected and documented.
Wholesale trade must meet many requirements to ensure proper quality of finished pharmaceutical products. In particular, these requirements are about transportation conditions, quality assurance, and storage.
Ensuring a proper quality is impossible without maintaining accurate documentation of every single process. But the thing is, routine documentation is traditionally completely handwritten, which provides for increased human error and further arising issues.
Supporting documentation must be instantly accessible/retrievable, unambiguous, detailed, and accurate. Control documents must be duly verified, dated, signed, with applied version control.
Good documentation according to state regulations
Internal processes managed by wholesale distributors should ensure that the wholesale trade of finished pharmaceutical products takes place in an appropriate manner, which isn’t adversely affecting their quality. Transportation conditions, proper packaging, temperature, humidity, and sanitization are the main conditions to control.
Among other, wholesale distributors must provide appropriate premises, utilities, and refrigeration equipment. In particular, these should be disinfected, maintained within the required temperature limits, and dry.
Medicinal products are sensitive to their transportation and storage conditions, in particular to temperature. Such temperature-sensitive medicinal substances include radiopharmaceuticals, vaccines, biologics, biotechnological products, and so-called combination products.
For better quality assurance, there are accredited requirements to the temperature range, which must be kept. It ranges from 2.0-8.0°C to 20.0-25.0°C for the routine storage of medicines.
There are state regulations by which both distributors in the United States and the European Union are guided. Such regulations specify the ideal conditions for transportation, further storage, and documentation of finished pharmaceutical products.
These include:
- USP <1083> Good Distribution Practices
- USP <1079> Good Storage And Shipping Practices
- USP <1118> Monitoring Devices – Time, Temperature, And Humidity
- FDA 21 CFR Part 203
- FDA 21 CFR Part 205
- Good Distribution Practices (GDP)
- European Pharmacopoeia, General notices
- Guideline on declaration of storage conditions for medicinal products particulars and active substances (CPMP/QWP/609/96 Rev. 2)
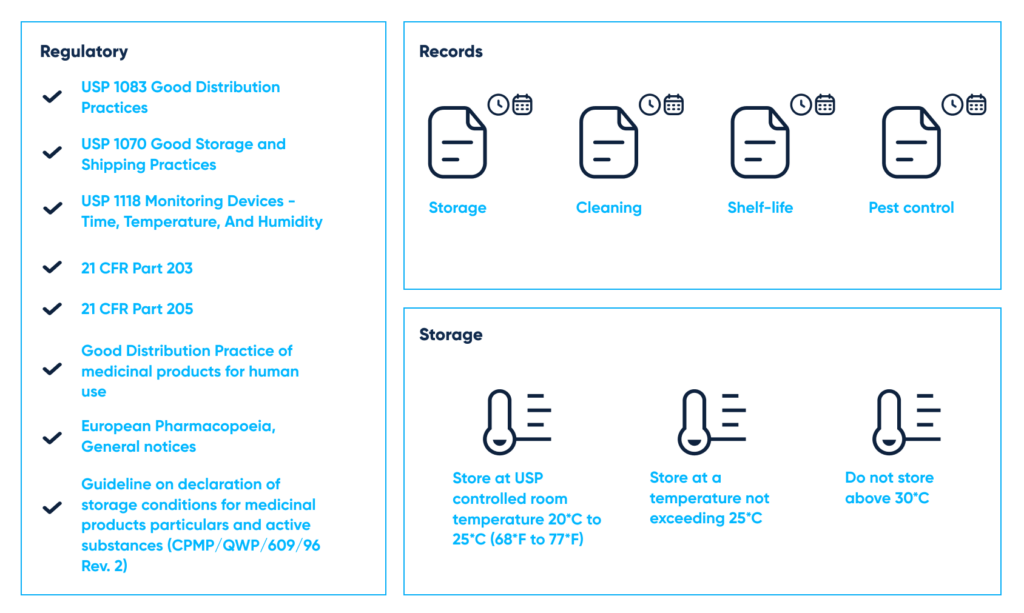
Guidelines for good documentation according to state regulations. By Abto Software
There are also regulations for mandatory supporting documentation as an important part of the whole process. They specify the principles of documentation, as well as requirements for accessibility, relevance, legitimacy, and also handwritten entries.
To dive in deeper, you can have a closer look at the infographic below:
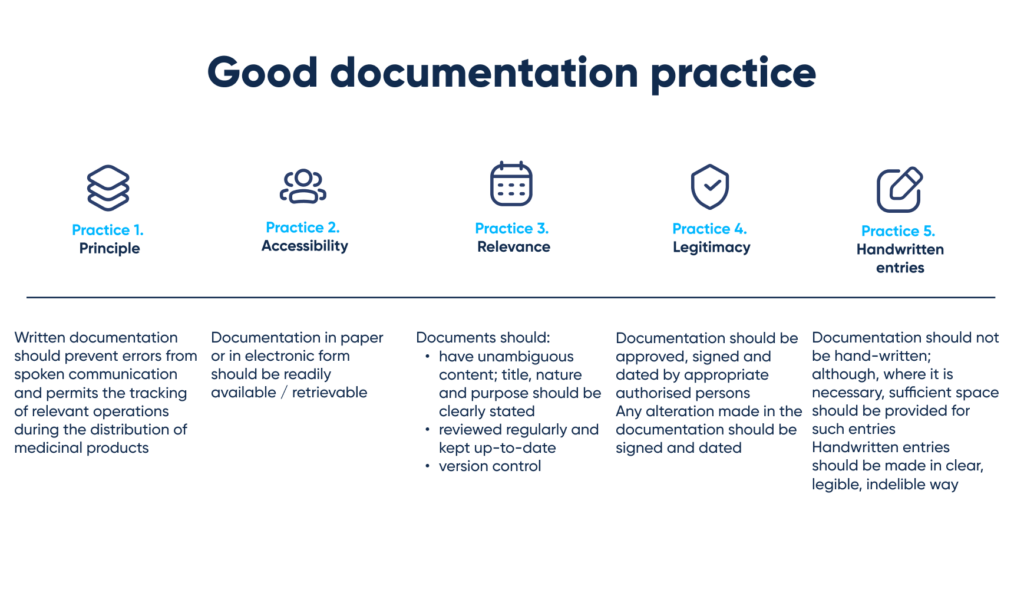
Practices of good documentation. By Abto Software
Cold Chain Good Practice
The monitoring and documentation of the transportation conditions, packaging integrity, proper sanitization, temperature, humidity, and other control parameters is essential to comply with the national regulations. Accurate tracking and documentation are crucial to ensure high quality, safety and therapeutic efficacy of the pharmaceutical products being distributed.
In order to comply with the WHO Technical Report Series No. 961 of 2011, Annex 9, the temperature in the storage premises should be recorded manually:
- At least twice a day, seven days a week in large storage locations
- At least once a day, five days a week in subnational storage locations
Example of annual temperature review report
The issue: A colossal data amount to handle
Wholesale distribution of finished pharmaceutical products does always come with supporting documentation. It might include records on shipment and arrival, temperature ranges, humidity levels, premise disinfection, and more.
Maintaining proper, accurate documentation is complicated in terms of both financial and human resources. Handwritten protocols increase risks, reduce accuracy and might negatively affect financial outcomes.
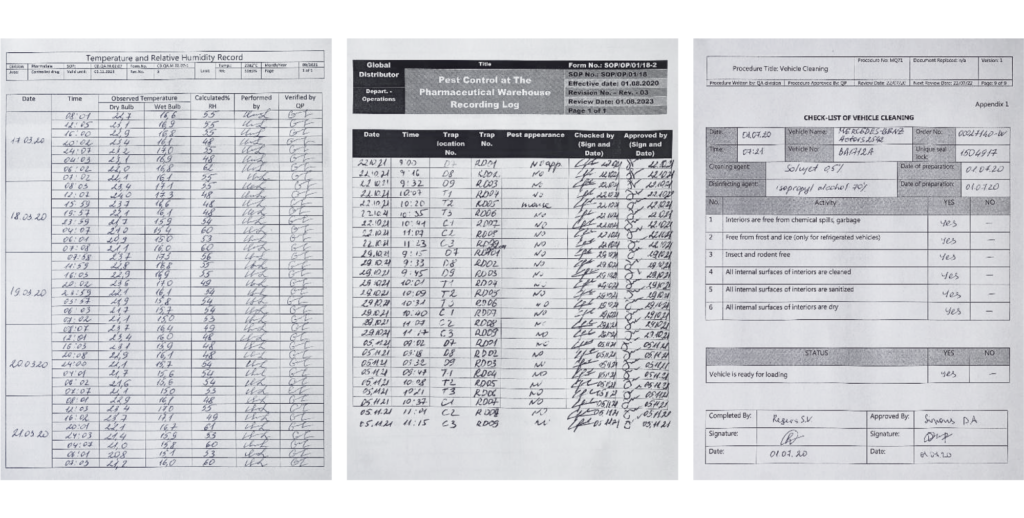
Example of manually filled-in reports
The solution: OCR technology to facilitate data management
As already mentioned above, pharmaceutical distribution does always come with supportive documentation. Inaccurate processing of the gathered information might complicate performance and productivity assessment, risk management, preventive and corrective actions.
To resolve this issue, we suggest adopting custom OCR software for simplified:
- Receipt control
- The condition of refrigerators or individual refrigerating chambers
- The compliance with established temperature ranges
- The integrity of seals
- The presence of inappropriate pharmaceutical products or other, non-pharmaceutical goods
- Quality control to inspect:
- Product integrity
- External damage
- Serial numbers and marking
- Expirations dates
- Storage control
- Temperature
- Humidity
- Location
- Sanitization
- Distribution control
- Correct packaging
- Equipment serviceability
Shelf life, demand forecasting, reverse distribution, and more
Wholesale distributors commonly require pharmaceutical products to have at least one year left of shelf life, and will only ship medicinal products that have at least six months shelf life remaining before actual expiration. Some dispensers even require pharmaceutical products to have more than six months before expiration.
Ensuring appropriate shelf life is important in terms of quality, but also in terms of smart resource allocation. But the thing is, this process, if carried out manually, is cost and time-consuming.
We believe Optical Character Recognition technology might be game changing in terms of examining shelf life. It might almost eliminate human error, increase accuracy, and allow responsible staff allocate valuable operational time more efficiently.
The concept is simple:
- The product is scanned upon receipt
- The product’s expiration date is recognized and transferred into the central database
- The system is configured to send immediate notifications under certain predefined conditions
- The manager is notified about product’s, which are potentially or already unfit for distribution
Process of information recognition and transferring
OCR technology can be also utilized to optimize:
- Demand forecasting and replenishment
- Reverse distribution
OCR software is the best solution to digitize supportive documentation associated with inventory assessment. With the right tools, managerial personnel can overview excessive products, understand trends, plan supplies, and organize reverse distribution in a much more cost-effective manner.
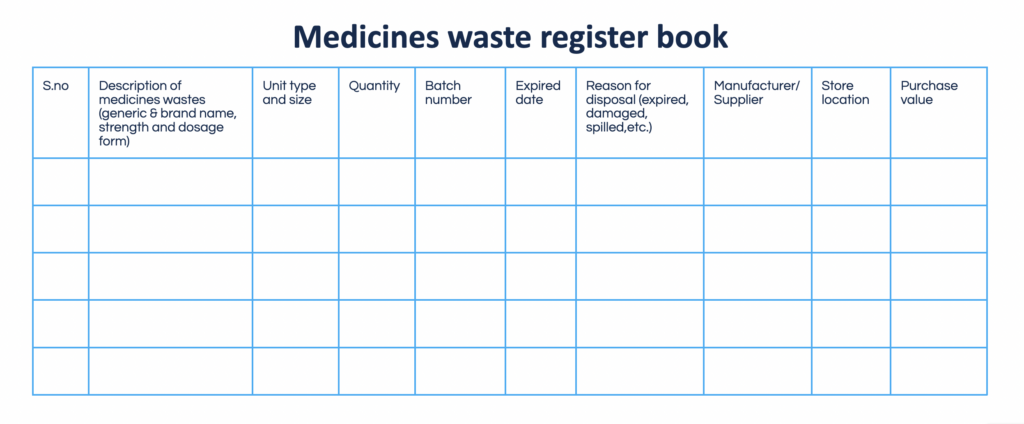
Medicines waste register book. By Abto Software
Digitized records for optimized business operation
Wholesale distributors face many industry-specific challenges, and most come with supportive documentation. As most control parameters are traditionally recorded manually, pharmaceutical distributors have to deal with unconsolidated data, human error, heavy workloads, dissatisfied staff, high risks, and potential financial losses.
A custom-designed OCR tool can be built to:
- Process documents with different, patchy layouts in one single batch
- Recognize tables
- Process low-quality, hard to read documents
- Process offset, rotated documents
This way, OCR software can help wholesale distributors organize day-to-day routine processes more efficiently. And with properly handled routine processes, pharmaceutical distributors can focus on more important issues.
So, why do we recommend implementing OCR software?
Reasons to implement OCR tool for facilitated drug distribution. By Abto Software
With the rights tool, you get:
- Instantly accessible, accurate records
- Up-to-date reviews
- Simplified monitoring of key control parameters
- Advanced analytics and reporting
Which provides:
- Data digitization
- Data integrity and legitimacy
- Optimized inventory
- Optimized workflows, demand forecasting, reverse distribution, and replenishment
- Risk elimination
- Cost efficiency
How we can help
Abto Software has the industry-specific expertise in custom software development to streamline your success. Engaging skilled software engineers, we leverage computational technology to automate complex processes, which are traditionally manual.
We know the specifics of the healthcare industry and ensure:
- HIPAA compliance
- NCPDP standard Medical Claim Form Handling
- Good documentation practice
- Good distribution practice
As well as compliance with other legislative regulations.
We leverage:
- Artificial Intelligence
- Computer Vision
- Business automation
- Chatbot solutions
- Prior Authorization
- Data Warehouses
To optimize:
- Data management
- Inventory management
- Drug design & development
- Clinical trials
- Drug track & trace to fight pharmaceutical counterfeiting
- Sanitary control
Ready to get started? Tell us your idea!


