Automation for drug production’ quality monitoring

Drug manufacturing is a meticulous process, which requires constant monitoring of critical control parameters. For different reasonable causes, pharmaceutical companies traditionally utilize handwritten documentation. Factory workers inspect indicators measured by mechanical equipment and update information manually. Human error, which might lead to data inconsistency, large investment and rather unreasonable workloads impacting the management’s awareness of the current state of production are just some of the problems, which may arise from sticking to handwritten documentation.
The problem
All processes associated with drug manufacturing include critical control parameters, which must be monitored at certain predefined intervals using special measuring equipment to maintain appropriate documentation. These commonly include temperature, humidity, pressure, and parameters of quality and quantity.
The monitoring, control, recording, and review of indicators is an integral part of the pharmaceutical business. This process is essential for batch release management, quality assurance, and an adequate time-to-market.
Some modern production equipment can be controlled electronically using custom-made software solutions. However, most production units do not allow engineers to monitor process indicators by using software tools, and require human surveillance, which allows line supervisors and managers to take immediate action.
What’s more, various important control parameters are monitored and recorded by different factory workers, which means, they can’t overview the overall process on other product lines and consolidate gathered data. That causes increased risks, decreased quality of the final product, longer time-to-market, and, accordingly, financial losses.
The solution
To digitize traditionally handwritten day-to-day documents, we suggest implementing custom OCR technology. By using optical character recognition systems to recognise production documents, pharmaceutical companies can both maintain their usual routines and optimize all processes related to data management and reporting.
The digitization of pre-designed, manually filled in protocols and other supporting documentation provides for:
- Data consolidation for improved data management (gathering, updating, and reviewing)
- Instant access to relevant real-time information for more thought-out decision-making
- Advanced analytics and reporting
- Good Manufacturing Practice and Good Documentation Practice compliance
The concept
The process of utilizing an integrated OCR tool for optimized drug development explained step-by-step:
- Factory workers responsible for recording critical control parameters scan the handwritten documents right after recording the measured indicators
- The integrated OCR algorithm recognizes and consolidates information
- The custom-built workflow platform processes the gathered information to update production statuses that indicate ongoing processes
- As soon as the workflow system detects inconsistencies using previously customized parameters, responsible employees receive an immediate notification with updated production statuses
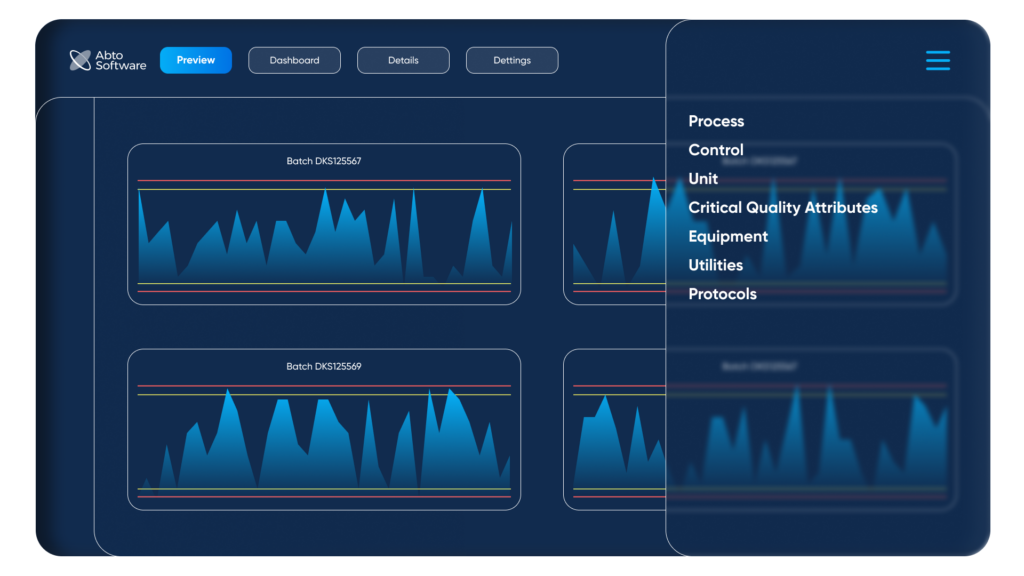
Preview of the menu for recording critical control parameters. By Abto Software
A custom workflow tool can include several sections such as status preview, dashboards, details, and settings, and can be used to organize:
- Process scheduling and observation
- Workflow monitoring
- Parameter monitoring
- In-process control
- Deep analytics
- Regular reporting

An interface of a custom workflow tool. By Abto Software
A platform with integrated OCR technology allows to:
- Scan and recognize handwritten supporting documentation, entry, update and review related data
- Optimize both data processing and reporting
- Set the control parameters to receive immediate notifications about both normal and abnormal states
- Response to received notifications in a timely manner to address emerging issues
Core features to implement might include:
- Production statuses
- Simultaneous observation of several production batches
- Text/vibro/sound notifications about both normal and abnormal events
- Regular reporting
Additional options
Apart from OCR technology, which can be implemented to process paper-based documentation, there are alternative options that might fit more flexible businesses.
Individual devices
One opportunity, which might potentially bring pharmaceutical companies even more business benefits, is to enter the updated indicators and notes into the system directly, thereby bypassing traditional record-keeping. To achieve such optimization, we suggest utilizing tablets or smartphones by every factory worker on site.
The pros:
- Greater accuracy
- Instant access to information in real-time
The cons:
- A change of the usual routine, which requires great flexibility to move to a modern practice
- A more complex process of correction if inconsistencies are allowed, which might require confirmation from assigned managerial personnel and potentially cause delays
- The need for devices to be easily disinfected to ensure sanitary compliance
Computer vision
We see another opportunity in the automatic scanning and tracking of indicators by integrated smart cameras. To achieve such automation, we suggest utilizing high-resolution smart cameras at every production line regularly scanning every indicator.
The pros:
- Greater accuracy
- Significant reduction of workload, which means cost efficiency and improved employee productivity
The cons:
- A transition to a new routine, which, again, might require great flexibility
- Initial investment
Internet of Things (IoT)
IoT is another opportunity, which provides pharmaceutical manufacturers with numerous business benefits. More specifically, IoT implementation might provide optimal conditions for handling bioactive materials, flawless operation of equipment, and assistance in prevention of counterfeiting.
The purposes IoT implementation should be considered include:
- Better control of the manufacturing environment
- Temperature
- Humidity
- Light
- Air
- Radiation
- Preventive maintenance of equipment
- Pressure gauges
- Air compressors
- Heat exchangers
- Multi-media filters
- Vacuum pumps
IoT’s most important feature is that it does not require human-to-human or human-to-computer interaction. Day-to-day operations can be conducted efficiently without constant human surveillance, which significantly reduces costs.
IoT networks include different smart tools that use sensors, processors, as well as other high-precision devices. These help to collect, consolidate and process data.
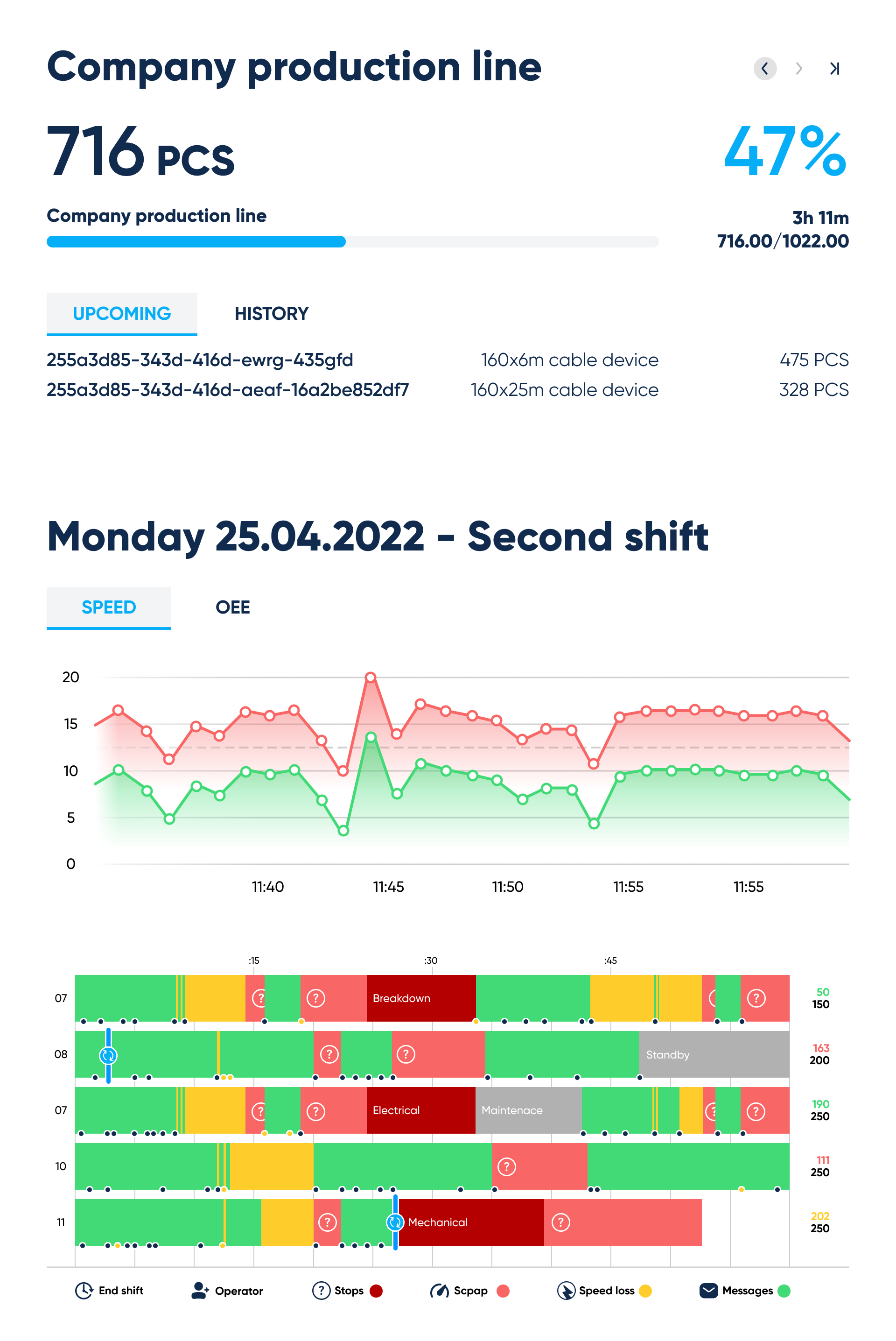
Production line interface. By Abto Software
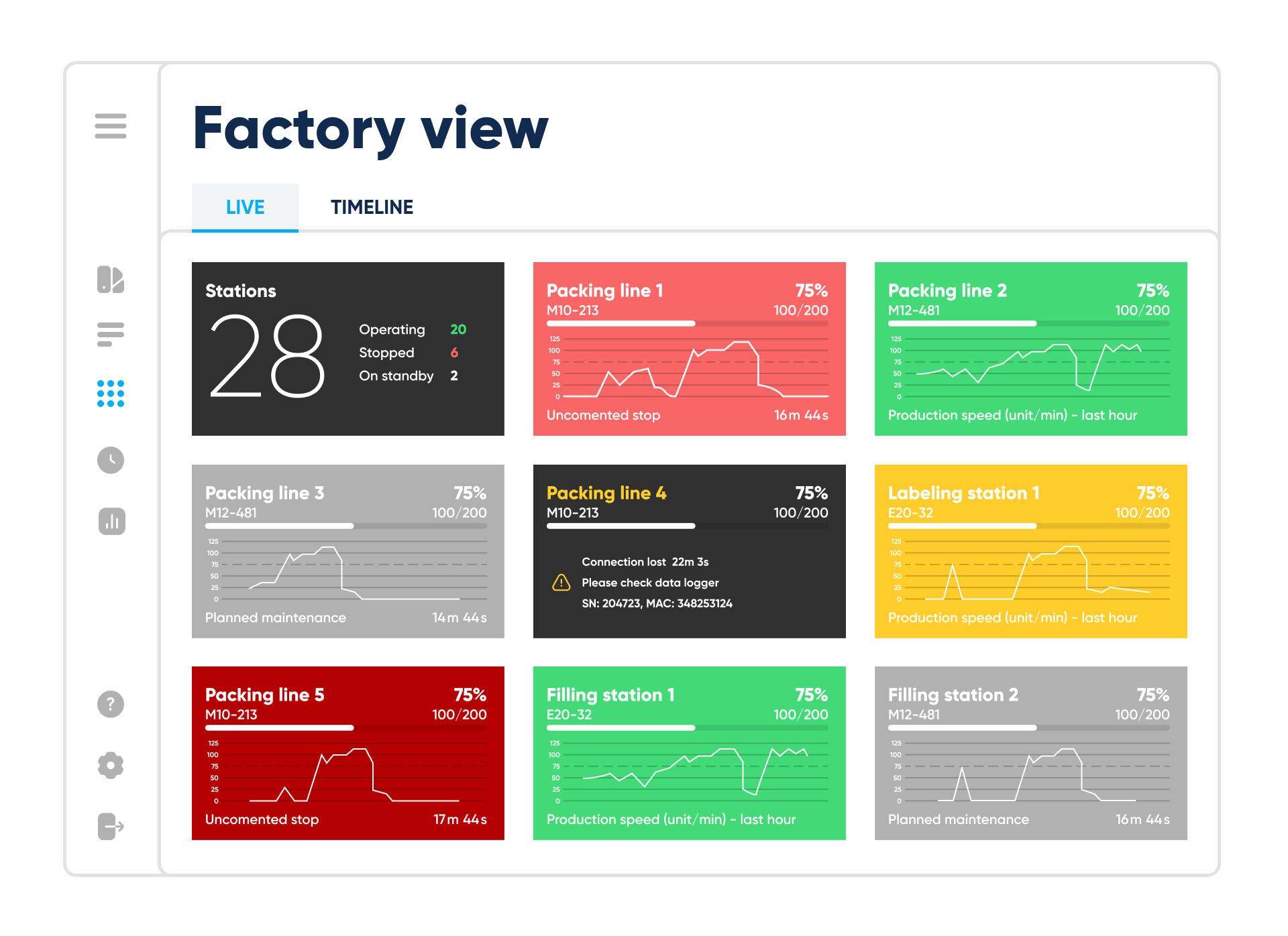
Factory view – live mode. By Abto Software
Business case
In the table below, we described the key process parameters for the polylactic acid manufacturing process. Talking about this specific business case, we see various benefits in implementing OCR technology to optimize the processing of the below mentioned supporting documentation.
Let’s look closer at the key control parameters and documentation produced during the process:
Pharmaceutical manufacturing requires every single process to be accurately monitored and documented. Accompanying documentation might include sampling plans, laboratory tests and results, quality standard justification documents, humidity and temperature records, batch records, control charts, and more.
The standard, manual process of documentation is slow and inefficient, and might even cause financial losses. To solve this issue, pharma businesses might adopt OCR technology to make the processing of documentation more productive.
Abto Software’s healthcare expertise
Abto Software delivers custom software development to assist business owners, medical workers, and patients. With over 16 years of experience, we make the most out of computational technology to help our clients improve productivity, decrease risks, increase profits, and remain highly competitive in a rapidly changing, demanding industry.
We deliver:
- Business automation (ERP & CRM platforms)
- Telemedicine and telehealth solutions (for example, smart chatbots)
- Predictive analytics (enterprise tools for discovering data patterns and making accurate predictions)
- Recommendation systems (EMR & EHR systems)
By leveraging:
- Artificial Intelligence (ML, DL & ANN)
- Computer Vision
- Optical Character Recognition (OCR)
- Blockchain technology
Contact us to get your custom-built OCR solution and optimize your production!


